Bill Rickard, co-founder and managing director of dabl
Dublin-based company dabl has formed a strategic alliance with Quintiles to provide ambulatory blood-pressure monitoring services in clinical trials. We talk to managing director Bill Rickard about how 24-hour blood-pressure monitoring is on the up.
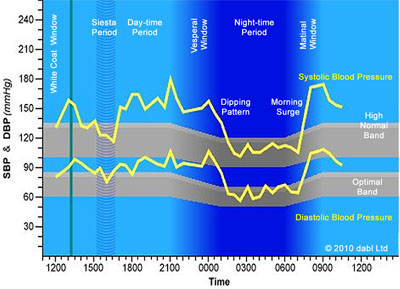
Many of us will, at some point, have had our blood pressure measured. In a doctor’s surgery it is all over within a couple of minutes. But that one reading offers just a snapshot of a person’s blood pressure. Instead, ‘ambulatory’ blood-pressure monitoring (ABPM) – where the person wears a device that takes periodic measurements, even during sleep – is a more informative option. It gives a more clinically relevant picture, particularly when one is asleep, when the healthy pattern is for blood pressure to dip.
High blood pressure is an important risk factor for a range of cardiovascular diseases, including stroke and heart attack and also cognitive impairment, explains Bill Rickard, co-founder and managing director of dabl.
For more than a decade, the Blackrock-based company has been developing and marketing software to analyse the results of ABPM, in hospitals, clinics, research centres, pharmacies and clinical trials. It now offers a suite of products and has just announced a strategic alliance with Quintiles, one of the largest clinical management companies in the world. In essence, it means Quintiles customers globally will have access to dabl’s round-the-clock ABPM services for clinical trials. “We are quite chuffed by that,” says Rickard. “It’s a reflection on the entire team here.”
Medical software – tight regulation
The medical software landscape has changed dramatically since he co-founded the company in 2000 with consultant cardiologist Prof Eoin O’Brien.
“We formed the company with a couple of people – you just couldn’t do that today because of all the regulatory requirements that are now required,” says Rickard. “Medical software is now a medical device, it is now under the umbrella of a whole new regulatory regime and if you don’t meet the regulations you will not be able to market your software.”
As well as developing software, dabl has also ensured its products comply with standards and it has been heavily involved in professional education about ABPM.
The company is now listed with the Food and Drug Administration (FDA), according to Rickard, and this opens the doors to being able to market its products and services, essentially worldwide.
Increasing demand for blood-pressure monitoring
Rickard has seen an increasing demand for ABPM, both in the clinic and in the community in recent years. And in 2008, dabl developed software for use in pharmacies, making it easier to bring ABPM into the community. It was a successful move.
“Literally week-on-week we are signing up new pharmacies in Ireland and abroad,” he says. “We have most of the major [pharmacy] groups in Italy and we have interest coming from various other countries, including the Cayman Islands and South Africa.”
More recently, dabl set up a clinical trials division within the company. “We had done a number of clinical trials and we felt there was a good market there, especially for blood pressure,” says Rickard.
He notes that the FDA in the US is interested in the potential for monitoring the cardiac side effects of drugs, and he anticipates that ABPM will become a more common component of clinical trials.
And they are well poised to catch that wave: just this month, dabl announced it had signed the agreement with Quintiles.
“That is significant because they are the biggest contract research organisation in the world,” says Rickard. “We went through a very strict audit procedure with them and they appointed us as their service provider for ABPM services. It’s a worldwide alliance.”
He credits the ‘team effort’ at dabl for the development, and more generally envisages that the 15-strong company will probably double its workforce in the next three to four years.
“We have designed [the software] in such a way that it is very scalable,” he says. “We are looking forward to a lot of growth from this in the future and it will be 99pc overseas.”
Siliconrepublic.com is hosting Med Tech Focus, an initiative which over coming months will cover news, reports, interviews and videos documenting Ireland’s leading role in one of the hottest sectors in technology.